Research Article- Asian Journal of Pharmaceutical Technology and Innovation(2018)
Mechanical Characterization of Extracellular Matrix Hydrogels: Comparison of Properties Measured by Rheometer and Texture Analyzer
Taraka Sai Pavan Grandhi1, Noreen Zaman2, Alamelu Banda3, Varsha Dhamankar2, Cathy Chu2, Emily Perotta2 and Irina Kadiyala2*2Pharmaceutical and Preclinical Sciences, Vertex Pharmaceuticals Incorporated, Boston, MA 02210, USA
3Pharmaceutical and Preclinical Sciences, Vertex Pharmaceuticals Incorporated, Presently at Genentech, San Francisco CA, 94080, USA
Irina Kadiyala, Pharmaceutical and Preclinical Sciences, Vertex Pharmaceuticals Incorporated, Boston, MA 02210, USA, Tel: +1 617-341-6100, Email: Irina_k@yahoo.com
Abstract
Extracellular matrix (ECM) hydrogels have shown remarkable benefit as new materials for regenerative medicine for multiple applications. However, for ECM-based materials to be used in vivo, they must possess appropriate mechanical properties to enable handling during storage and administration, as well as properties to induce the needed biological responses. The experiments carried out in this study allowed deeper understanding of the physical characteristics of gels towards their use in a clinical setting and subsequent commercialization. Using rheometer and texture analyzer, we expanded the previously reported mechanical characterization of ECM gels and here we present data on the new tests such as constant shear at different temperatures; cross-over temperature/gel point; cohesivity, adhesivity and hardness; fitting to Burger’s model; and strain-stiffening. We compared the mechanical properties of ECM hydrogels to collagen hydrogels, the latter were used as an internal control. Mechanical properties of ECM hydrogels were measured during different stages of hydrogel formation (pre-gel, gelation and formed hydrogel). Overall, gel complex modulus measured using rheometer correlated with hardness measured using texture analyzer across measured hydrogel concentrations, indicating that the measurements done with rheometer and texture analyzer were complementary to each other. In addition, two model drugs with poor solubility and similar molecular weight, but differing in charge were incorporated into the UBM ECM hydrogel to study the rheological behavior of the drug-hydrogel composites. The presence and properties of the drugs were found to have a moderate effect on hydrogel mechanical properties depending on the charge of the drug.
Keywords
Extracellular matrix (ECM) hydrogels, Rheometer, Texture analyzer
Introduction
Hydrogels are formed from hydrophilic, water insoluble polymeric matrices that swell under aqueous conditions [1] and exhibit a wide range of mechanical properties. They have been shown to be safe and biocompatible, and have generated much interest in the burgeoning field of regenerative medicine [2]. Unlike polymerizing systems that require the addition of external catalysts, copolymerizing agents or photo polymerizing compounds to initiate gelation, some naturally occurring gels can undergo rapid physical gelation upon a change in environmental conditions. Thermosensitive hydrogels, which include naturally occurring hydrogels composed of collagen or extracellular matrix (ECM), undergo phase transition from liquid to solid in response to a change in temperature, and have drawn particular interest due to their vast potential applications. These hydrogels are thermoplastic and can be injected as liquids via commonly used gauge needles at an injury site; an increase in temperature to 37°C will cause them to gel in-situ, thus making them a valuable tool for minimally invasive drug delivery [3-5].
Whole ECM gels consist of a complex mixture of glycosaminoglycans (GAGs), non-structural proteins and structural proteins such as collagen, fibronectin, laminin and elastin, each of which has shown some regenerative capacity [6-9]. ECM gels are gaining traction in research and the clinic due to their higher regeneration potential, e.g. for CNS regeneration [10-13]. ECM gels promote regeneration by recruitment of endogenous stem and progenitor stem cells from the surrounding tissues [14]. Soluble proteins in the ECM matrix create a chemotactic gradient for the stem cells to migrate, whereas insoluble proteins provide a structural and mechanical framework for the cells to differentiate upon [14]. ECM gels characterized in this study were derived from porcine urinary bladder (UBM ECM) and have shown immense benefit in tissue regeneration in multiple animal models, pre-clinical trials and small human clinical studies [15-18]. In addition to providing chemical signals, the mechanical properties of the hydrogels have been found to be important in regulating the biological responses [5,19,20]. For example, materials with elastic modulus of about 0.5 MPa have been shown to stimulate neuronal stem cells to mature into neurons for regeneration [21,22] and be used for the treatment of stroke [23]. Or ECM gels with elastic stiffness of 0.4-0.9 MPa have been shown to have varying regenerative capacity in the liver depending on formulation composition [24].
In-depth understanding of hydrogel mechanical properties can aid in the optimization of hydrogel preparation and improve their administration in the clinic. While ECM hydrogel viscoelastic properties by rheology have been published previously in multiple papers [5,6,25], we expanded our understanding of the mechanical properties using novel techniques such as constant shear at different temperatures, cross-over temperature or gel point, cohesivity, adhesivity, and hardness, and strain-stiffening. In addition, while there are studies showing the use of rheometer [25] and limited studies showing the use of texture analyzer for mechanical characterization of ECM hydrogels (yield stress, strain, gelation kinetics, etc.) [6], there are no studies providing a direct comparison between the characterization by rheometer and texture analyzer. We present procedures for characterization of UBM ECM hydrogels using these two instruments that offer complementary data and tools. Multiple rheological and texture properties of the hydrogel were measured under surface-parallel shear forces and normal forces respectively. Furthermore, ECM hydrogel results from both techniques (rheological and texture based) were compared to that of collagen gels, which have been used extensively by other researchers and provide an internal reference. In addition, we determined the impact of drug loading on the mechanical properties of the ECM drug-hydrogel composite using two model drugs, glipizide and tamsulosin, and identified properties of the drugs that may impact gel formation. Incorporation of drugs into the hydrogel can provide additional therapeutic benefit when administered at the site of injury. The work presented here might provide a platform for the study of UBM ECM, collagen or novel hydrogels for therapeutic use.
Experimental Section
Materials
Powdered urinary bladder matrix (UBM) was provided by the Stephen Badylak Laboratory, McGowan Institute of Regenerative Medicine, University of Pittsburgh (Pittsburgh, PA, USA). FibriCol Bovine Collagen, Type I (10 mg/mL) was obtained from Advanced BioMatrix (Carlsbad, CA, USA). Pepsin, Pluronic F127 and glipizide were obtained from Sigma-Aldrich (St. Louis, MO, USA). Tamsulosin was obtained from Selleck Chemicals (Houston, TX, USA). 10x phosphate buffered saline (PBS) was prepared in-house at Vertex Pharmaceuticals and diluted to 1x PBS with water. Nanopure water was used for all preparations unless specified. Stress controlled Discovery Hybrid Rheometer-1 from TA instruments (New Castle, DE, USA) was used in rheological measurements. The TA.XTPlus Texture Analyzer (Stable Micro Systems, Godalming, UK) was used for texture analysis.
Preparation of UBM ECM and Collagen Pre-Gel Solutions
Detailed description of ECM pre-gel solution preparation is presented in Freytes et al. [6]. In brief, 100 mg of lyophilized UBM ECM powder was mixed with 10 mg of pepsin in 10 mL of 0.01 N HCl for 48 h at room temperature. The pre-gel solution was prepared by an acid neutralization method. The stock solution was diluted with 1X PBS and neutralized with 0.1 N NaOH to pH 7.2-7.4. Collagen pre-gels were also prepared by acid neutralization with 0.1 N NaOH and 1x PBS as per the protocol of the vendor (Advanced BioMatrix) [26]. UBM ECM gel and collagen gels were prepared at 2, 4 and 8 mg/mL for rheological and texture analyzer characterization described below.
Two small molecule model drugs (glipizide and tamsulosin) sparingly soluble in water were incorporated into the UBM ECM gels. The chosen drugs have similar molecular weight (~400 Da) and logP (~2.8), but vary in charge. Glipizide is negative at neutral pH, and tamsulosin is positive at neutral pH. These drugs were incorporated into 4 mg/mL UBM ECM hydrogels at 25 and 50 wt%/wt%. To eliminate the potential effect due to differences in particle size, the drugs were screened and a particle size fraction of 106 to 212 μm was used for these experiments for both drugs. Each drug (25 or 50% by total weight) was mixed with Pluronic F127 (6.25% by total weight) and the drug/Pluronic F127 mixture was subsequently combined with the UBM ECM stock solution (4 mg/mL). The drug-ECM pre-gel mixture was then neutralized as described above.
Methods
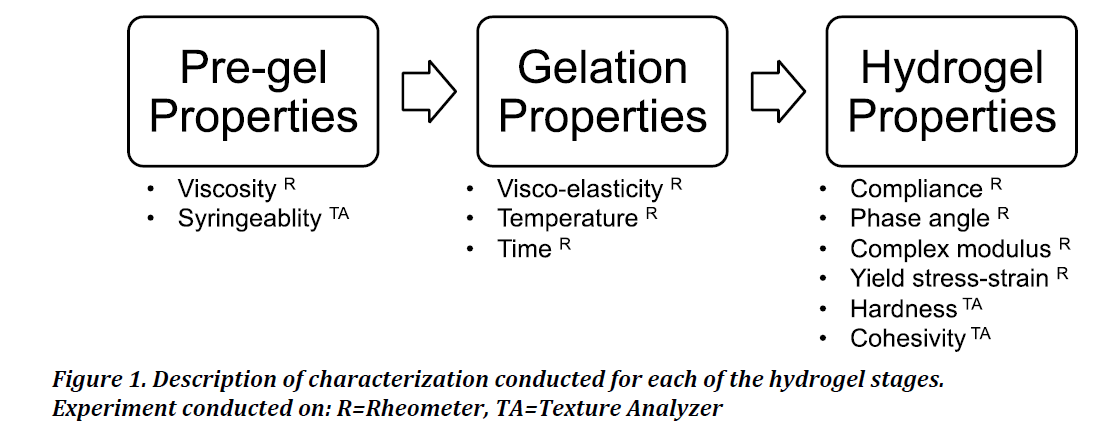
Figure 1 details the various characterizations carried out at the different phases of gel formation and specifies the instrument used for the respective measurements. For all rheometer tests, measurements were initiated by adding 800 μL of pre-gel solution to the plate and cone (1.995°) geometry on the TA Instruments DHR-1 series rheometer. The bottom plate of the rheometer was maintained at 4°C until the start of the experiment. The vendor guidelines were followed for various experiments that were carried out unless specified [27]. Gel and hydrogel terminology are used interchangeably. All terminology used in this study is consistent with the TA manual [27].
Pre-gel solution properties
The UBM ECM and collagen pre-gel solutions were characterized by rheometer for viscosity, and by texture analyzer for syringeability.
Viscosity: The viscosity of the pre-gel solutions was determined by constant shear and shear ramp methods. For constant shear measurements, the viscosity was measured at 4°C and 10°C at 1 Pa shear for 5 min. For shear ramp measurements, the pre-gels were subjected to a shear ramp from 1-1000 1/s. The shear ramping experiment was conducted at 4°C since the pre-gel solution gels at the higher temperatures. The Herschel-Bulkley equation was fitted to the data to determine flow indices.
Syringeability: Syringeability (resistance to extrusion) was measured on the UBM ECM and collagen pre-gel solutions. Syringeability was measured by applying a normal force on the syringe piston using a flat probe connected to the TA.XTPlus instrument. Approximately 0.5 mL of solution was extruded from a 1½" 21G needle with a 1mL syringe at a rate of 1 mm/s The mean force produced was recorded with a detection limit set at 0.5 g of force.
Gelation properties
The gel point and temperature-responsiveness of the materials were determined on the rheometer.
Gel Point: The time required for gelation, which is defined as the gel point, was measured by tracking the storage modulus (G’) of the gel. The gelation process was studied for the UBM ECM and collagen gels on the rheometer using two methods. In the first method (flash method), the temperature was increased from 4°C to 37°C in less than one minute after introduction of the pre-gel to the rheometer. The pre-gels were exposed to a strain of 0.1% with a constant angular frequency of 1 rad/s.
Temperature-responsive gelation test: In the second method (ramp method), the pre-gel solutions were exposed to a strain of 0.1% with a constant angular frequency of 1 rad/s while the temperature was gradually ramped from 4°C to 37°C at a rate of 0.33°C/min. The storage modulus (G’) and loss modulus (G”) were tracked and the time where G’=G” was determined to be when gelation occurred.
Hydrogel properties
Dynamic mechanical analysis was carried out on the formed UBM ECM and collagen hydrogels on the rheometer to determine the storage, loss and complex moduli, phase angle, yield strain, and yield stress. The hardness, cohesivity and adhesivity of both gels were determined on the texture analyzer.
Dynamic mechanical analysis: Storage modulus (G’), loss modulus (G”), complex modulus (G*) and phase angle (tan δ) were measured by applying a strain of 0.1% with an angular frequency of 1 rad/s.
Yield strain was measured by amplitude sweep, which involves applying a strain from 0.001 to 100% at a constant angular frequency of 1 rad/s. A logarithmic exponential decrease in the storage modulus was determined to be the yield strain (%) value.
Yield stress was measured by creep method, which involves applying incremental stress until strain recovery was incomplete. The procedure was as follows. A constant stress of 0.1 Pa was initially provided for 60 s followed by a recovery time of 540 s. The constant stress was ramped up 1.5 fold with each repeat across 20 repeats. Yield stress was measured as the stress value at which the strain (%) after recovery was incomplete and approximately equal to or greater than 0.5% strain.
Burger’s model was used to fit the creep data to calculate strain under prolonged stress conditions. Constant stress creep measurements were performed to compare the predicted strain values from the model to the measured values for the rheometer.
Hardness, cohesivity and adhesivity: Approximately 750 μL UBM ECM or collagen pre-gel solution were cast in 2 mL vials for 90 min at 37°C. The gels were impinged with a cylindrical rod on the TA.XTPlus Texture Analyzer at a rate of 1 mm/s up to a distance of 5 mm into the gel. Force measurement was initiated after reaching the trigger force of 0.5 g. Initial peak of the force time curve was noted as the hardness peak, area under the positive region of the curve as cohesivity and area under the negative region of the curve as adhesivity.
Statistical Analysis
One-way ANOVA was used to compare the different gel properties at three different concentrations. Pairwise comparisons were performed using Tukey’s multiple comparisons test (Tukey-Kramer method) to understand the differences between individual concentrations. Significance was defined as p<0.05.
Conclusion
This study provides a detailed mechanical assessment of viscoelastic properties of UBM ECM gels by rheometer and texture analyzer. The tests were conducted for various stages of hydrogel formation in order to understand nuances of the system behavior as a function of its stages and to aid in proper handling for in vivo studies. The study was conducted for specific UBM hydrogel and corresponding studies should be done for the select systems. We determined that:
- For all concentrations of UBM ECM gels, rapid gelation was observed around 25°C with a gel point of less than 5 min.
- Gel complex modulus measured using rheometer correlated with hardness measured using texture analyzer, indicating that the measurements done with rheometer and texture analyzer were complementary to each other.
- Yield stress and yield strain were negatively correlated and gels were found to undergo strain stiffening at lower concentrations.
- The collagen gels in comparison to the UBM ECM gels had higher pre-gel viscosity, modulus, hardness and gelation time. The collagen gels also showed a greater degree of strain stiffening than the UBM ECM gels.
- Insoluble drugs were loaded into the UBM ECM gels, and impacted the mechanical properties of the gels depending on the charge of the drug.
Although, a specific hydrogel UBM system was used in this study the recommendation to utilize both techniques due to their complementary nature can be extended to other hydrogel systems. This would yield a more comprehensive understanding of mechanical properties of systems and would allow for better development decisions.
Acknowledgment
We thank Vertex Pharmaceuticals Incorporated for funding this research work. We would like to thank Dr. Stephen Badylak’s group at McGowan Institute of Regenerative Medicine, University of Pittsburgh for their expertise with ECM gels and also for supplying the UBM ECM used in this study. We would also like to thank TA instruments and Texture Technologies for their technical support with the rheometer and the texture analyzer, respectively.
References
- Kashyap N, Kumar N, Kumar M, Hydrogels for pharmaceutical and biomedical applications, Crit Rev Ther Drug Carrier Syst, 22(2); 107-49, 2005.
- Slaughter B, Khurshid S, Fisher O, Khademhosseini A, Peppas N, Hydrogels in regenerative medicine, Adv Mater, 21(32-33); 3307-3329, 2009.
- Yang J, Yeom J, Hwang B, Hoffman A, Hahn S, In situ-forming injectable hydrogels for regenerative medicine, Progr Polym Sci, 39(12); 1973-1986, 2014.
- Prestwich G, Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine, J Control Release, 155(2); 193-199, 2011.
- Medberry CJ, Crapo PM, Siu BF, Carruthers CA, Wolf MT, Nagarkar SP, Agrawal V, Jones KE, Kelly J, Johnson SA, Velankar SS, Watkins SC, Modo M, Badylak SF, Hydrogels derived from central nervous system extracellular matrix, Biomaterials, 34(4); 1033-1040, 2013.
- Freytes D, Martin J, Velankar S, Lee A, Badylak S, Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix, Biomaterials, 29(11); 1630-1637, 2008.
- Dao M, Hashino K, Kato I, Nolta J, Adhesion to fibro nectin maintains regenerative capacity during ex vivo culture and transduction of human hematopoietic stem and progenitor cells, Blood, 92(12); 4612-4621, 1998.
- Rooney J, Gurpur P, Yablonka-Reuveni Z, Burkin D, Laminin-111 restores regenerative capacity in a mouse model for α7 integrin congenital myopathy, Am J Pathol, 174(1); 256-264, 2009.
- Geiger M, Li R, Friess W, Collagen sponges for bone regeneration with rhBMP-2, Adv Drug Deliv Rev, 55(12); 1613-29, 2003.
- Frantz C, Stewart K, Weaver V, The extracellular matrix at a glance, J Cell Sci, 123(24); 4195-4200, 2010.
- Busch S, Silver J, The role of extracellular matrix in CNS regeneration, Curr Opin Neurobiol, 17(1); 120-127, 2007.
- Barros C, Franco S, Müller U, Extracellular matrix: functions in the nervous system, Cold Spring Harb Perspect Biol, 3(1); a005108, 2011.
- Fawcett J, The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease, Prog Brain Res, 218; 213-226, 2015.
- Wolf MT, Daly KA, Brennan Pierce EP, Johnson SA, Carruthers CA, Amore A, Nagarkar SP, Velankar SS, Badylak SF, A hydrogel derived from de-cellularized dermal extracellular matrix, Biomaterials, 33(29); 7028-7038, 2012.
- Badylak SF, Kochupura PV, Cohen IS, Doronin SV, Saltman AE, Gilbert TW, Kelly DJ, Ignotz RA, Gaudette GR, The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium, Cell Transplant, 15; 29-40, 2016.
- Kimmel H, Rahn M, Gilbert T, The clinical effectiveness in wound healing with extracellular matrix derived from porcine urinary bladder matrix: A case series on severe chronic wounds, J Am Col Certif Wound Spec, 2(3): 55-9, 2010.
- Afaneh C, Abelson J, Schattner M, Janjigian YY, Ilson D, Yoon SS, Strong VE, Esophageal reinforcement with an extracellular scaffold during total gastrectomy for gastric cancer, Ann Surg Oncol, 22(4); 1252-1257, 2015.
- Parcells A, Abernathie B, Datiashvili R, The use of urinary bladder matrix in the treatment of complicated open wounds, Wounds, 26(7); 189-196, 2014.
- Wang J, Thampatty B, An introductory review of cell mechanobiology. Biomech Model Mechanobiol, 5(1); 1-16, 2006.
- Badylak S, Freytes D, Gilbert T, Extracellular matrix as a biological scaffold material: Structure and function, Acta Biomater, 5(1); 1-13, 2009.
- Li X, Katsanevakis E, Liu X, Zhang N, Wen X, Engineering neural stem cell fates with hydrogel design for central nervous system regeneration, Prog Polym Sci, 37(8); 1105-1129, 2015.
- Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE, Substrate modulus directs neural stem cell behavior, Biophys J, 95(9); 4426-4438, 2008.
- Ghuman H, Massensini AR, Donnelly J, Kim SM, Medberry CJ, Badylak SF, Modo M, ECM hydrogel for the treatment of stroke: Characterization of the host cell infiltrate, Biomaterials, 91; 166-181, 2016.
- Klaas M, Kangur T, Viil J, Mäemets-Allas K, Minajeva A, Vadi K, Antsov M, Lapidus N, Järvekülg M, Jaks V, The alterations in the extracellular matrix composition guide the repair of damaged liver tissue, Sci Rep, 6(6); 27398, 2016.
- Baker E, Bonnecaze R, Zaman M, Extracellular matrix stiffness and architecture govern intracellular rheology in cancer, Biophys J, 97(4); 1013-1021, 2009.
- Ulrich T, Jain A, Tanner K, MacKay J, Kumar S, Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices, Biomaterials, 31(7); 1875-1884, 2010.
- Instructions for TA HR-2 Shear Rheometer.
- Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D, Substrate compliance versus ligand density in cell on gel responses, Biophys J, 86(1); 617-628, 2004.
- Ehrbar M, Sala A, Lienemann P, Ranga A, Mosiewicz K, Bittermann A, Rizzi SC, Weber FE, Lutolf MP, Elucidating the role of matrix stiffness in 3D cell migration and remodeling, Biophys J, 100(2); 284-293, 2011.
- Cheng G, Tse J, Jain R, Munn L, Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells, PLoS One, 4(2); e4632, 2009.
- Engler A, Sen S, Sweeney H, Discher D, Matrix elasticity directs stem cell lineage specification, Cell, 126(4), 677-689, 2006.
- Yan C, Altunbas A, Yucel T, Nagarkar R, Schneider J, Pochan D, Injectable solid hydrogel: Mechanism of shear-thinning and immediate recovery of injectable β-hairpin peptide hydrogels, Soft Matter, 6(20); 5143-5156, 2010.
- Liang Y, Walczak P, Bulte J, The survival of engrafted neural stem cells within hyaluronic acid hydrogels, Biomaterials, 34(22); 5521-5529, 2013.
- Li Y, The mechanism of collagen self-assembly: Hydrophobic and electrostatic interactions, Thesis, University of Florida, 2009.
- Oegema T, Laidlaw J, Hascall V, Dziewiatkowski D, The effect of proteoglycans on the formation of fibrils from collagen solutions, Arch Biochem Biophys, 170(2); 698-709, 1975.
- Ruggeri A, Benazzo F, Collagen-proteoglycan interaction, Ultrastructure of the Connective Tissue Matrix, Springer US, 113-25, 1984.
- Yang Y, Kaufman L, Rheology and confocal reflectance microscopy as probes of mechanical properties and structure during collagen and collagen/hyaluronan self-assembly, Biophys J, 96(4); 1566-1585, 2009.
- Buehler M, Nanomechanics of collagen fibrils under varying cross-link densities: atomistic and continuum studies, J Mech Behav Biomed Mater, 1(1); 59-67, 2008.
- Arevalo R, Urbach J, Blair D, Size-dependent rheology of type-I collagen networks, Biophys J, 99(8); L65-L7, 2010.
- Motte S, Kaufman L, Strain stiffening in collagen I networks, Biopolymers, 99(1); 35-46, 2013.
- Dolz M, Hernández M, Delegido J, Creep and recovery experimental investigation of low oil content food emulsions, Food Hydrocoll, 22(3); 421-7, 2008.
- Martucci J, Ruseckaite R, Vazquez A, Creep of glutaraldehyde-crosslinked gelatin films, Mater Sci Eng, 435; 681-686, 2006.
- Valentin J, Stewart-Akers A, Gilbert T, Badylak S, Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds, Tissue Eng Part A, 15(7); 1687-1694, 2009.
- Guarnieri D, Battista S, Borzacchiello A, Mayol L, De Rosa E, Keene DR, Muscariello L, Barbarisi A, Netti PA, Effects of fibronectin and laminin on structural, mechanical and transport properties of 3D collageneous network, J Sci Mater Med, 18(2); 245-53, 2007.